38 warning labels on drugs
Who Reads the Drug Warning Labels? - Hormones Matter Perhaps it is time that we start demanding that drug warning labels mean something. They should accurately and completely reflect the real dangers associated with each and every drug. Frequency of adverse events should be noted on the warning labels. This is not too much to ask for. Patients, doctors and everyone else involved should insist on it. Pharmacy, Medication Warning Labels & Stickers | PDC Never get important medications or directions mixed up ever again with these pre-printed warning labels for medical, pharmacy, and lab applications. ... Communication Label (Paper, Permanent) Drug Discontinued 1-9/16" x 3/8" Yellow - 500 per Roll, 2 Rolls per Box . 1-378. $8.11. Add to Cart.
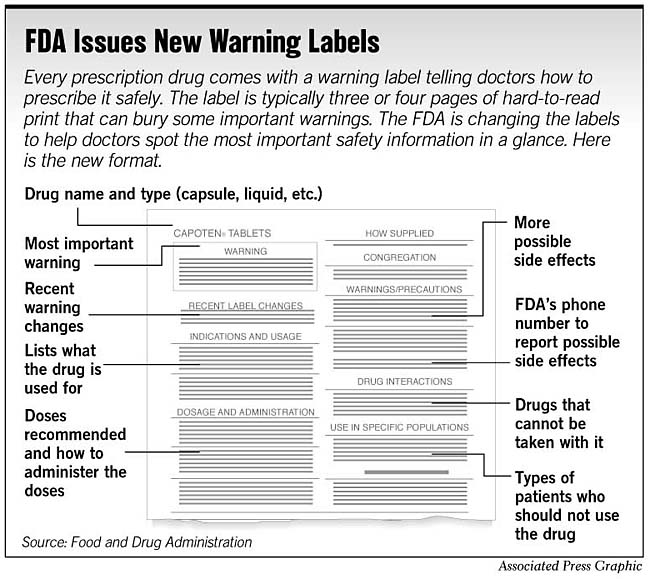
Do Drug Labels Overdo Warnings? - WebMD "These are 20,000- to 30,000-word massive documents." Warnings on Drug Labels In 2006, the FDA set rules to make drug labels easier to read and understand and reduce "overwarning" on drug...
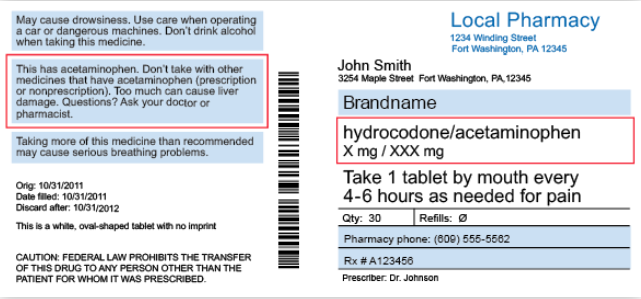
Warning labels on drugs
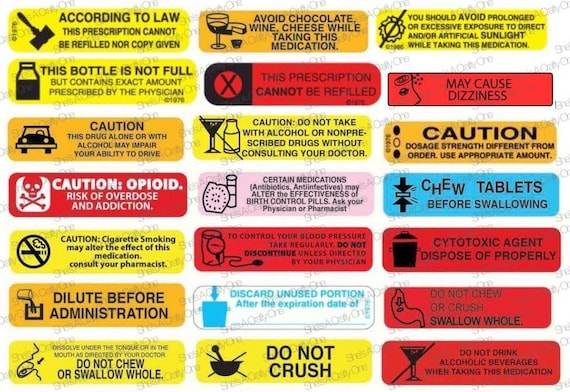
Warning Labels on High-Powered Magnets Unlikely to Prevent Child ... FDA Alerts Warning Labels on High-Powered Magnets Unlikely to Prevent Child Injuries MONDAY, Oct. 3, 2022 -- Parents of children with high-powered magnet exposures often do not know if warning labels were present, or they report not reading them, according to a study published online Oct. 3 in Pediatrics. Understanding the FDA's Black Box Warnings on Drug Labels Boxed warnings, also known as black box warnings, are the most serious type of warning issued by the Food and Drug Administration (FDA). These warnings are front and center on a drug's... A Warning About Warning Labels - U.S. Pharmacist Label Color: Approximately 42% of the patients correlated the label's color to the severity of the message. Patients reported that a red label meant danger, yellow translated to caution, and blue, white, and green labels were viewed as "recommendations" that were not as critical as the instructions on red labels.
Warning labels on drugs. Warning Label Templates - Customizable Templates | Avery.com Prop 65 Warning Label Templates. If you make or sell products in California you may need to include a p65 warning on your products. Choose the right template below to fit your products, customize & order. 1" x 2-5/8" Rectangle Label. 1" x 4" Rectangle Label. 2/3" x 3-7/16" Rectangle Label. 2" x 3" Rectangle Label. 1" x 2-5/8" Rectangle Label. What Is A FDA Black Box Warning Label - NastLaw The most prescribed class of antibiotics - fluoroquinolones (Cipro, Levaquin and Avelox) - have a black box warning for serious nerve damage and tendon rupture. Popular heartburn drugs including Nexium, Prilosec and Prevacid, have a black box warning for several side effects. Patients and doctors rely on proper warning labels on drugs. Perceptions of prescription warning labels within an underserved ... Prescription warning labels may still be a valid method of communicating medication information, as long as they are clear and easily comprehensible to patients; however, proper counseling is needed to point out the information on the warning label for those populations who may not regard them. Warnings and Precautions, Contraindications, and Boxed Warning Sections ... This guidance is intended to assist applicants and reviewers in drafting the WARNINGS AND PRECAUTIONS, CONTRAINDICATIONS, and BOXED WARNING sections of labeling, as described in the final rule...
› drugs › drug-safety-and-availabilityFDA adds Boxed Warning for risk of serious injuries caused by ... Feb 18, 2022 · Drowsiness is already listed as a common side effect in the drug labels of all insomnia medicines, along with warnings that patients may still feel drowsy the day after taking these products. Stronger Warning Labels Needed on Drugs That Make Patients Lose Control ... June 29, 2016. Stronger Warning Labels Needed on Drugs That Make Patients Lose Control. Public Citizen Petitions the FDA for Black Box Warnings on Drugs That Cause Pathological Gambling, Hypersexuality, Compulsive Shopping or Eating, Other Uncontrollable Behaviors › drugs › understanding-over-counterSunscreen: How to Help Protect Your Skin from the Sun | FDA Nov 08, 2021 · The labels are required to state whether the sunscreen remains effective for 40 minutes or 80 minutes when swimming or sweating, and all sunscreens must provide directions on when to reapply ... USP 800 Labeling Requirements | United Ad Label Shelf labels help communicate that vital information to your staff. In addition, precaution labels applied to the container alert workers to the hazardous drug and its potential harm. Use Appropriate Personal Protective Equipment (PPE) An essential USP <800> safety element is the use of appropriate PPE.
Controlled Substance Labels - Free Shipping | LabelValue Medical Labels Morphine Hydrocodone (Vicodin) Hyrdomorphone (Dilaudid) Oxycodone (OxyContin, Percocet) Meperidine (Demerol) Fetanyl Methadone Methamphetamine Adderall Ritalin Ketamine Anabolic steroids Buprenorphine (Suboxone) Codeine and hydrocodone products Alprazolam (Xanax) Clonazepam (Klonopin) Diazepam (Valium) Lorazepan (Ativan) Defective Drug Warning Labels and Off-Label Use - lawinfo.com An adequate warning label for a drug will include information such as dosage, active ingredients, and known harmful side effects. However, it's often the case a drug is prescribed by a doctor for a use that wasn't intended, or hasn't been publically endorsed by the drug developers. This was the case with Fen-Phen. › tobacco-products › products-guidanceLabeling and Warning Statements for Tobacco Products | FDA FDA has provided a compliance date of August 10, 2018, for the required health warning statements on packages and advertisements for "covered" tobacco products (except cigars and pipe tobacco 1 ... Stanton Introduces Legislation to Require Warning Labels on Addictive ... The bill would amend the Controlled Substance Act to require the Food and Drug Administration to issue regulations to require warning labels be added directly to the opioid prescription bottle that states that the drugs may cause "dependence, addiction, or overdose." This is similar to what is required for cigarette packaging.
What Information Should Be on Drug Labels? - MedicineNet The label's side panel instructs on using this drug product, as well as how to store it to ensure that it remains effective in the future. There could be a warning label associated with storage and handling. Lot numbers Manufacturers assign lot numbers to products, which can be used as a reference if adverse reactions occur.
Warning Label Lawsuits | LegalMatch A warning label lawsuit is a lawsuit brought by a consumer of a product. Consumer products include food, drink, drugs, electronic devices, and mechanical devices. Federal law requires product suppliers and manufacturers to provide adequate warning of the dangers the product may pose. Warnings are in the form of a label that describes the dangers.
Black Box Warning - StatPearls - NCBI Bookshelf Boxed warnings (formerly known as Black Box Warnings) are the highest safety-related warning that medications can have assigned by the Food and Drug Administration. These warnings are intended to bring the consumer's attention to the major risks of the drug. Medications can have a boxed warning added, taken away, or updated throughout their tenure on the market. Over 400 different ...
The Importance of Reading Medication Warning Labels But some warning signs can have dire results if ignored, particularly the warnings on medication labels. "May cause drowsiness and dizziness; do not operate heavy machinery while taking this medication" is a common warning label on prescription pain killers. You might read that and think "I don't operate a bulldozer, so I should be fine."
en.wikipedia.org › wiki › Boxed_warningBoxed warning - Wikipedia The FDA can require a pharmaceutical company to place a boxed warning on the labeling of a prescription drug, or in literature describing it. It is the strongest warning that the FDA requires, and signifies that medical studies indicate that the drug carries a significant risk of serious or even life-threatening adverse effects.
› newsNews: latest stories, exclusives, opinion & analysis - mirror The latest UK and World news, from Mirror Online. Find the best stories, opinion, pictures and video on the day's events.
Pharmaceutical Labeling 101: FDA Regulations Guide Manufacturers commonly use multi-layer labels since they need to fit a lot of text in a small area. Ideally, labels that can stand chemical exposure, UV light, and moisture are suitable for such tasks. Laminated sheet labels and freezer stickers are also widely accepted. Effect of COVID-19 on Pharmaceutical labeling
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA cautions about using ... The testosterone product labels have been updated. The revised labels clarify the approved uses of these medications and include information about a possible increased risk of heart attacks and ...
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
Black Box Warning List of Medications | FADIC Black Box Warning 2022 List of Medications from FADIC 1- What is a black box warning? 💊 2- Black Box and Off-Label Uses ... Black box warnings are the most severe for prescription medications imposed by the Food and Drug Administration (FDA). Additionally, It highlights potentially fatal, life-threatening, or disabling adverse effects of ...
FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels Black Box Warning for Xanax (Anxiety) Xanax is a benzodiazepine drug. It 's also used to manage phobic behaviors and specific conditions like social anxiety. Prescription rates in the last 18 years have risen only 67%, but the number of Xanax related deaths per year has multiplied sixfold.
Prescription Labels and Drug Safety - Consumer Reports Warnings typed directly onto patient labels in a large typeface. Research has found that fewer than 10 percent of people examine their drug containers for the colorful warning stickers that...
FDA's Generic Drug Warning Labeling Rule Change Late last year, the Food and Drug Administration proposed new regulations that would authorize the makers of generic drugs to update safety labels without waiting for F.D.A. approval, to warn of newly discovered risks. The change is strongly opposed by the manufacturers of generic drugs out of fear of being held liable for damages to patients ...
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning.
› drugs › drug-safety-and-availabilityFDA identifies sudden discontinuation of opioid pain medicines FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering
Custom Labels That Support Good Health Outcomes - Drug Package Prescription Warning Labels Are Now Available! Protect your customers from potential hazards with prescription warning labels from Drug Package. Shop Warning Labels. Drug Package World Headquarters. 901 Drug Package Lane O'Fallon, MO 63366 Phone (800) 325-6137 Fax (800) 600-6137.
FDA Label Search - Food and Drug Administration You can search for labels by drug name and link to the Library's information resources about marketed drugs. Download All Labels Health information suppliers and others can download all of...
446 Drug Warning Label Stock Photos - Dreamstime Download Drug Warning Label stock photos. Free or royalty-free photos and images. Use them in commercial designs under lifetime, perpetual & worldwide rights. Dreamstime is the world`s largest stock photography community.
Misunderstanding Drug Warning Labels - Medscape Purpose: The common causes for misunderstanding prescription drug warning labels (PWLs) among adults with low literacy were studied. Methods: A total of 74 patients reading at or below the sixth ...
A Warning About Warning Labels - U.S. Pharmacist Label Color: Approximately 42% of the patients correlated the label's color to the severity of the message. Patients reported that a red label meant danger, yellow translated to caution, and blue, white, and green labels were viewed as "recommendations" that were not as critical as the instructions on red labels.
Understanding the FDA's Black Box Warnings on Drug Labels Boxed warnings, also known as black box warnings, are the most serious type of warning issued by the Food and Drug Administration (FDA). These warnings are front and center on a drug's...
Warning Labels on High-Powered Magnets Unlikely to Prevent Child ... FDA Alerts Warning Labels on High-Powered Magnets Unlikely to Prevent Child Injuries MONDAY, Oct. 3, 2022 -- Parents of children with high-powered magnet exposures often do not know if warning labels were present, or they report not reading them, according to a study published online Oct. 3 in Pediatrics.


















![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/2-Figure1-1.png)






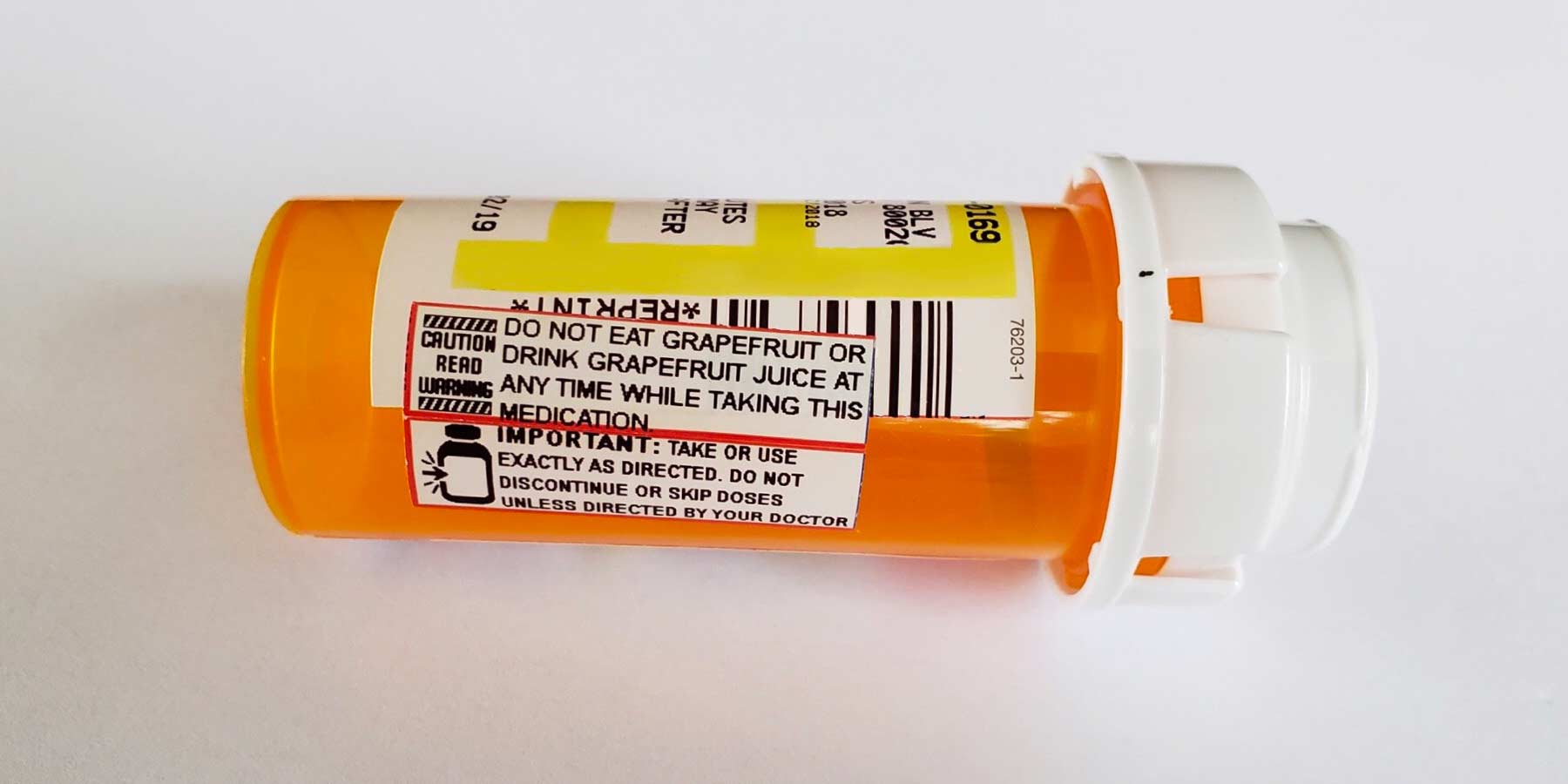

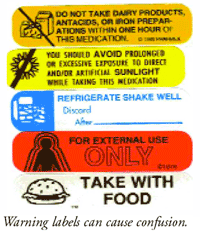

Post a Comment for "38 warning labels on drugs"