41 fda approved health claims on food labels
Pet Food | FDA - U.S. Food and Drug Administration For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ... Label Claims for Conventional Foods and Dietary Supplements | FDA there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the...
Questions and Answers on Health Claims in Food Labeling | FDA 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that authorizes the use of a health claim about the relationship between soy protein and the reduced risk of coronary heart disease.

Fda approved health claims on food labels
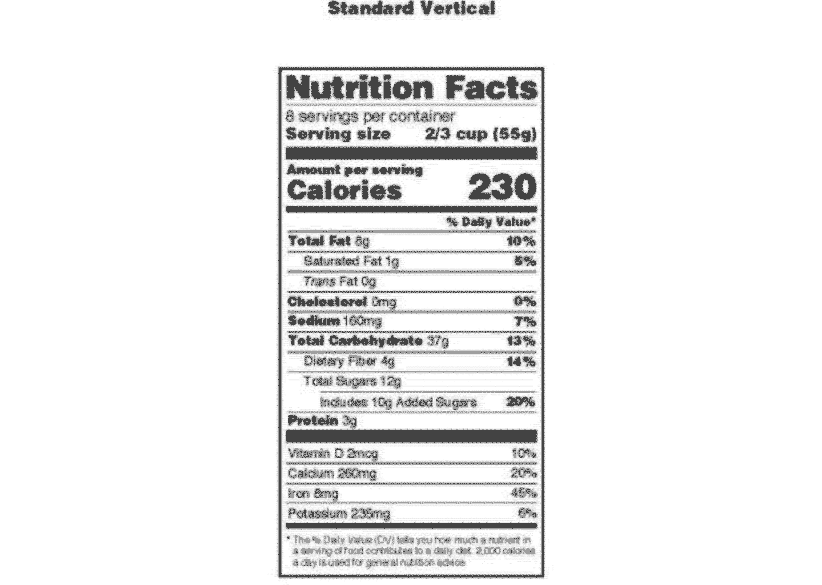
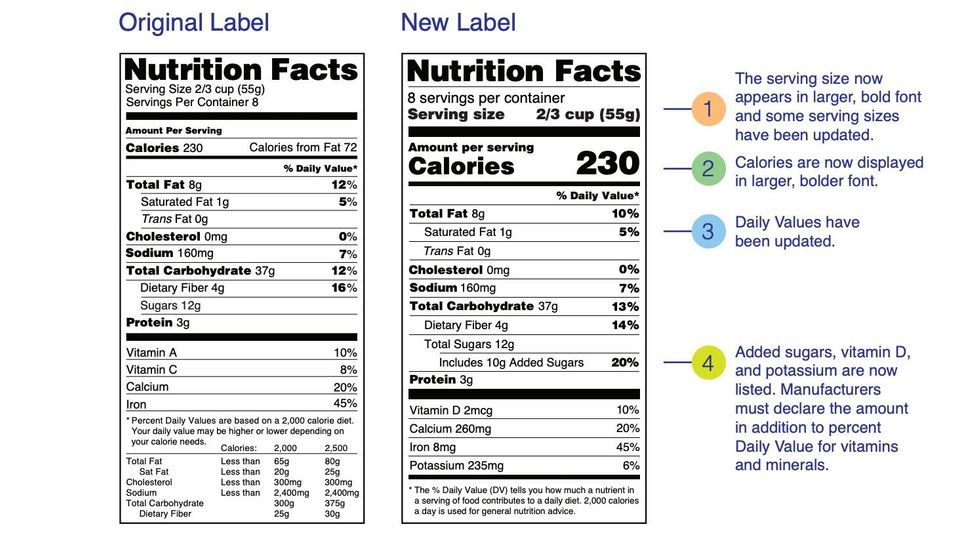
Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ... Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim"
Fda approved health claims on food labels. Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient... Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
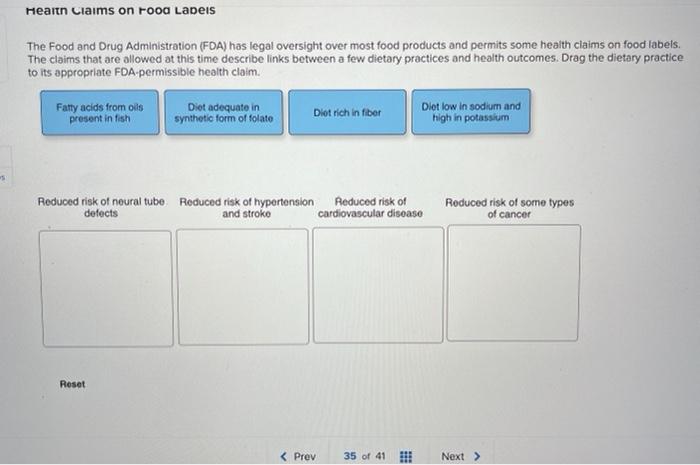
Everything you need to know about Health Claims on Food Labels The "qualified" health claims. The authorized health claims by the FDA must have significant scientific agreement among qualified experts to support the scientific evidence for a substance - disease relationship. The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and ... Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ... Additional Information about High-Intensity Sweeteners FDA approved acesulfame potassium for use in specific food and beverage categories in 1988 (53 FR 28379), and in 2003 approved it as a general purpose sweetener and flavor enhancer in food, except ... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are...
CFSAN Constituent Updates | FDA - U.S. Food and Drug Administration Sep 08, 2022 · 9/8/2022 - FDA Announces Targeted Sampling, Additional Efforts to Enhance the Safety of Leafy Greens 9/1/2022 - September 2022 is the 24th National Food Safety Education Month 8/24/2022 - FDA ... Health Claims on Food Labels - Consumer Reports Specifically, grass-fed meat and dairy has a more healthful ratio of omega-6 polyunsaturated fatty acids to omega-3s. Too much omega-6 fat in your diet can cause inflammation, which may be a ... Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and Is It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
COVID-19 Vaccines | FDA - U.S. Food and Drug Administration The FDA announced in a letter of enforcement discretion that it does not intend to object to the use of certain qualified health claims regarding the consumption of magnesium and a reduced risk of ...
FDA perspectives on health claims for food labels - PubMed The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims charact …
Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Understanding Health Claims on Food Labels - Food Smart Colorado There are a total of 12 health claims approved by the FDA. A complete listing of health claims approved for food labels is available here: #2- Nutrient content claims describe a food and the level of a particular nutrient in that food. "Low fat," and "High fiber" are both examples of nutrient content claims. For a table showing nutrient ...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food...
Questions and Answers on Dietary Supplements | FDA Oct 26, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ...
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient...
Qualified Health Claims: Letters of Enforcement Discretion | FDA Letter Updating the Qualifying Level of Oleic Acid for the Oleic Acid From Edible Oils and Coronary Heart Disease (Corbion Biotech Petition) Qualified Health Claim July 16, 2020. Folic Acid ...
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim...
Health Claims on Food Labels: LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim"
Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ...


:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)






























Post a Comment for "41 fda approved health claims on food labels"